
Evusheld
AstraZeneca,Britain
$2688.00-
SelectSpecification/Number
-
ParameterDetailed information
-
NoticeCustoms clearance instructions
-
Required readingConsumption notice
- Description
- Information
Pre-exposure prophylaxis
EVUSHELD is indicated for the pre-exposure prophylaxis of COVID-19 in adults and adolescents aged 12 years and older weighing at least 40 kg
Treatment
EVUSHELD is indicated for the treatment of adults and adolescents (aged 12 years and older weighing at least 40 kg) with COVID-19, who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19
Posology and method of administration
Posology
Pre-exposure prophylaxis The recommended dose in adults and adolescents aged 12 years and older weighing at least 40 kg is 150 mg of tixagevimab and 150 mg of cilgavimab, administered as two separate sequentialintramuscular injections.There are no safety and efficacy data available on repeat dosing.TreatmentThe recommended dose in adults and adolescents aged 12 years and older weighing at least 40 kg is 300 mg of tixagevimab and 300 mg of cilgavimab (Table 1), administered as two separate sequential intramuscular injections.EVUSHELD should be given as soon as possible after a positive viral test for SARS-CoV-2 and within 7 days of the onset of symptoms of COVID-19.
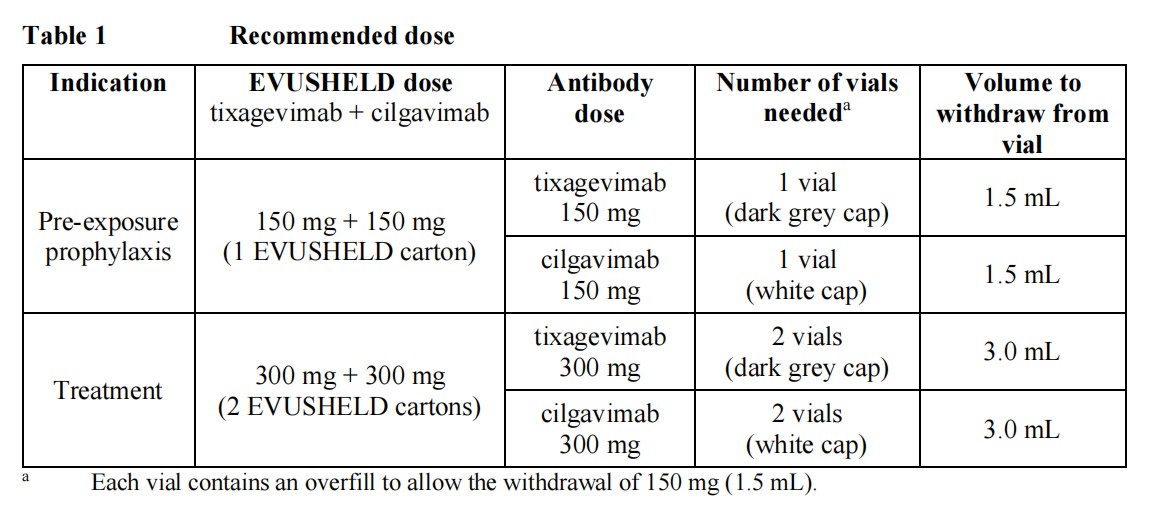
Elderly No dose adjustment is requiredRenal impairment No dose adjustment is requiredHepatic impairment No dose adjustment is required Special warnings and precautions for useHypersensitivity including anaphylaxisSerious hypersensitivity reactions, including anaphylaxis, have been observed with monoclonal antibodies. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue administration and initiate appropriate medicinal products and/or supportive therapy. Cardiovascular and/or thrombo-embolic eventsIn the PROVENT study, participants in the EVUSHELD arm experienced more serious cardiovascular adverse events compared to those in the placebo arm (0.7% versus 0.3%), notably coronary events (e.g myocardial infarction). A smaller imbalance has been observed for serious thrombo-embolic events (0.5% versus 0.2%). The majority of subjects had cardiovascular risk factors and/or history of cardiovascular disease that could explain the occurrence of such events. A causal relationship between EVUSHELD and these events has not been established.
The risks and benefits should be considered prior to initiating EVUSHELD in individuals at high risk for cardiovascular or thrombo-embolic events. Patients should be advised of signs or symptoms suggestive of cardiovascular event (notably chest pain, dyspnoea, malaise, feeling lightheaded or faint) and to seek immediate medical attention if such symptoms occur.
Clinically significant bleeding disorders As with any other intramuscular injections, EVUSHELD should be given with caution to patients with thrombocytopenia or any coagulation disorder. Antiviral resistance The clinical trials with EVUSHELD were conducted when Alpha, Beta, Gamma and Delta variants were predominant. Efficacy of tixagevimab and cilgavimab against some circulating SARS-CoV-2 variants with decreased in-vitro susceptibility is uncertain.Based on clinical data from PROVENT, the duration of protection following administration of a single EVUSHELD dose (150 mg of tixagevimab and 150 mg of cilgavimab) is estimated to be at least 6 months. Due to the observed decrease in in-vitro neutralisation activity against the Omicron subvariants BA.1, BA.1.1 (BA.1+R346K), BA.4 and BA.5 the duration of protection of EVUSHELD for these subvariants is currently not known. COVID-19 vaccinesPre-exposure prophylaxis with EVUSHELD is not a substitute for vaccination in individuals for whom COVID-19 vaccination is recommended.
Evusheldinformation
No information yet!!!